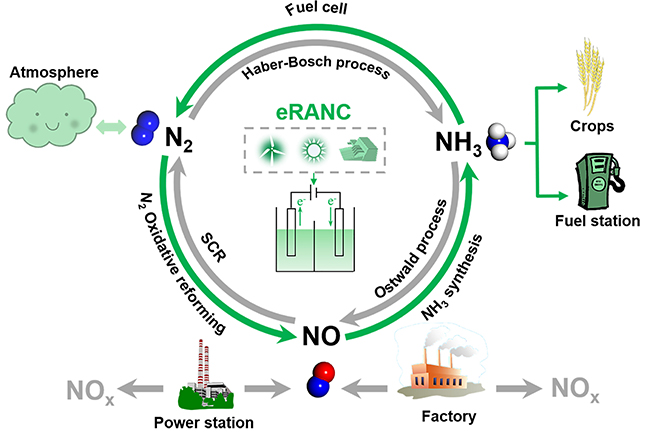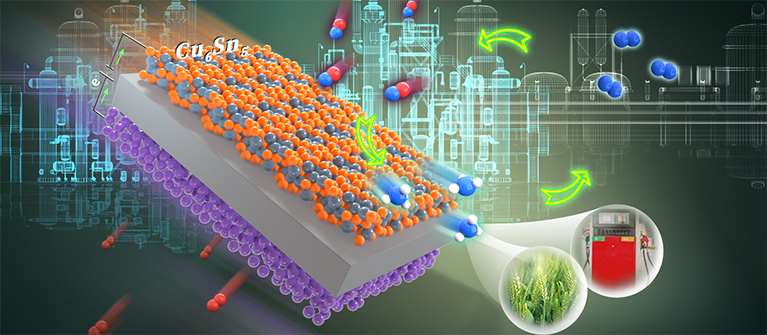
Alternative routes to the Haber–Bosch process are being sought to electrify ammonia synthesis. Nitric oxide can be electrocatalytically converted into ammonia, but the Faradaic efficiencies and rates of production are currently far below those needed for industrial application. Here we report a rationally designed copper–tin alloy that is highly active in the synthesis of ammonia from nitric oxide. The rate of ammonia production in a flow cell reached 10 mmol cm−2 h−1 with a Faradaic efficiency of >96% at a current density >1,400 mA cm−2, and it remained stable at >600 mA cm−2 with an ammonia Faradaic efficiency of ~90% for 135 h. The rate of ammonia production in a scaled-up electrolyser comprising a membrane electrode assembly reached ~2.5 mol h−1 with a current of 400 A at ~2.6 V. We attribute the high ammonia production rate to the enhanced intrinsic activity of the alloy; the kinetic barriers of protonation are invariably low over a range of Cu6Sn5-derived surface structures.