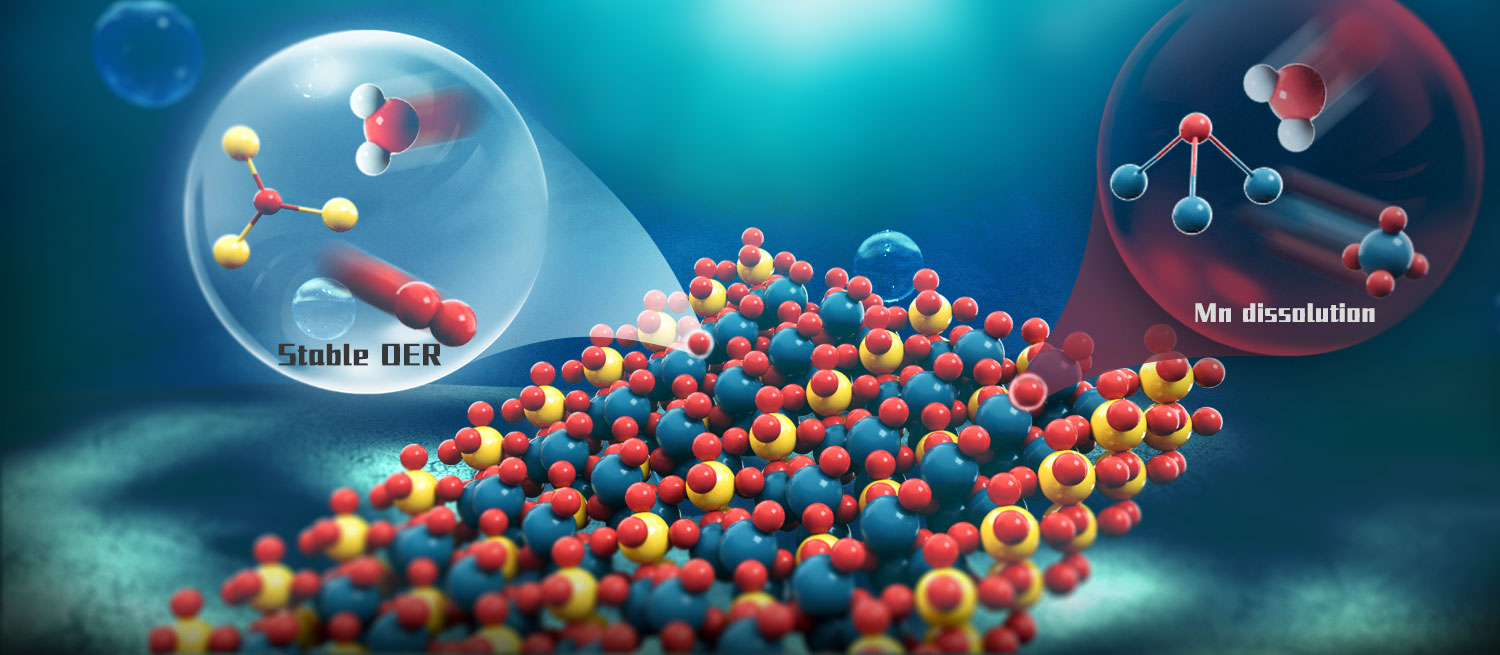
Earth-abundant, acid-stable catalysts for the oxygen evolution reaction are essential for terawatt-scale hydrogen production using proton exchange membrane (PEM) electrolysers. Here we report that optimizing the lattice oxygen structure of manganese oxide allows it to sustain the oxygen evolution reaction for over one month at 1,000 mA cm−2 in 1 M H2SO4. The lifetime enhancement was achieved by substituting pyramidal oxygen with planar oxygen, which has a stronger Mn–O bond and thus suppresses the dissolution of manganese ions. Calculations show that the lattice oxygen dissolution is the bottleneck of deactivation, and this process is less favourable by over 0.2 eV on planar oxygen compared with pyramidal oxygen. Our material shows excellent performance even in a PEM electrolyser, reaching 2,000 mA cm−2 at 2 V with durability exceeding 1,000 h at 200 mA cm−2. This study expands the potential of Earth-abundant catalysts for PEM electrolysis, which may mitigate the reliance on iridium.