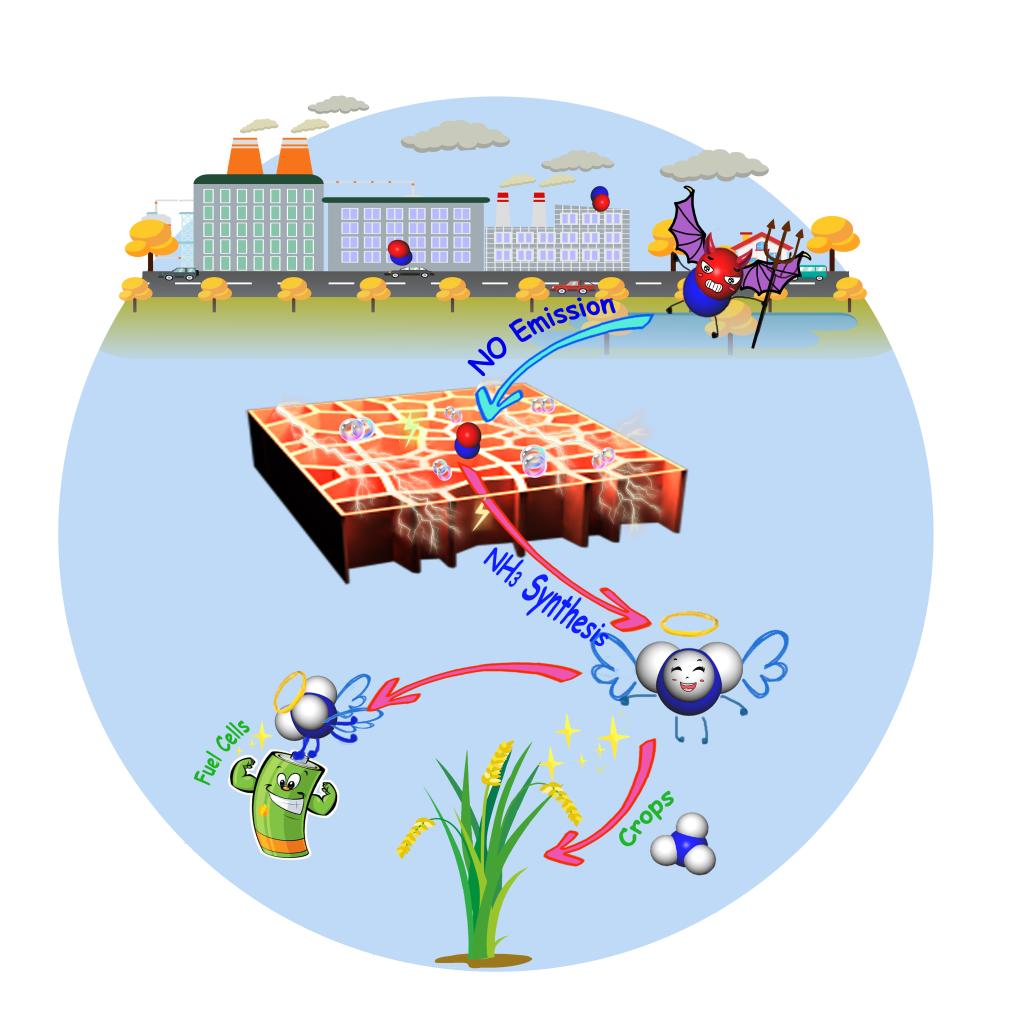
NO removal from exhausted gas is necessary due to its damage to environment. Meanwhile, the electrochemical ammonia synthesis (EAS) from N2 is suffering from a low reaction rate and Faradaic efficiency (FE). Herein, we propose an alternative route for ammonia synthesis from exhausted NO via electrocatalysis. Density functional theory calculations indicate electrochemical NO reduction (NORR) is more active than N2 reduction (NRR). Via a descriptor‐based approach, Cu was screened out to be the most active metal catalyst for NORR to NH3 due to its moderate reactivity. Moreover, kinetic calculations reveal NH3 is the most preferred product relative to H2 , N2O and N2 on Cu. Experimentally, a record‐high EAS rate of 517.1 μmol·cm‐2 ·h‐1 and FE of 93.5% were achieved at ‐0.9 V vs. RHE using a Cu foam electrode, exhibiting stable electrocatalytic performances with 100 hours run. The present work provides an alternative strategy to EAS from exhausted NO, coupled with NO removal.