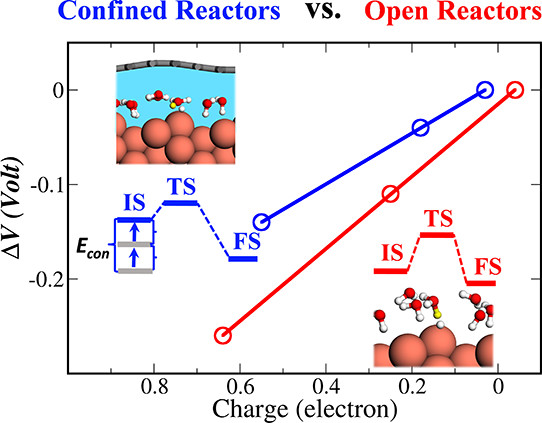
A number of experiments have demonstrated that electrochemical reactions are feasible in confined nanoscale reactors, while what the fundamentals of confined electrochemistry are is not clear. Using first-principles calculations and electrochemical modeling, we find that the capacitance in the confined nanoscale reactors can be significantly enhanced, compared to an open electrode interface, essentially promoting the electrochemical reactions and charge transfer efficiency in nanoscale reactors. More importantly, this is a general character, as found in a variety of electrochemical and thermochemical reactions. At the end, we use the recently defined new concept of “confinement energy” for understanding the nature of confined electrochemistry from both thermochemical and electrochemical points of view.